Aluminum
General Information
The third most abundant element in the earth’s crust, aluminum (Al) is, nevertheless, a relatively new metal to the human race. The commercial process allowing its recovery economically was not discovered until around the turn of the 20th century. Aluminum metal is silvery white, has a low specific gravity (2.69) and a low melting temperature. Except for iron and steel, aluminum is probably used for more purposes than any other metal. Its light weight – about one-third that of steel – and its strength when alloyed with other metals are two reasons for aluminum’s versatility. Other reasons are the ease with which the metal can be cast, machined, rolled, forged, extruded, and drawn. It has high electrical conductivity and significant resistance to atmospheric corrosion. Aluminum paint, beverage cans, baseball bats, high-voltage power lines, house siding, boats, and airplanes are just a few examples of aluminum metal's use.
Another advantage of aluminum is that it can be efficiently recycled. Whereas the extraction of new aluminum from bauxite consumes huge amounts of energy (this includes the energy consumed during mining, processing the ore, and land reclamation), aluminum metal reclaimed from used products requires only 5 percent as much energy. Much of the feedstock of the aluminum metal industry now consists of used beverage cans. In 1991, nearly 57 billion used aluminum cans were recycled in the United States.
Mineralogy and Processing of Arkansas Bauxite

Pisolitic bauxite
.jpg)
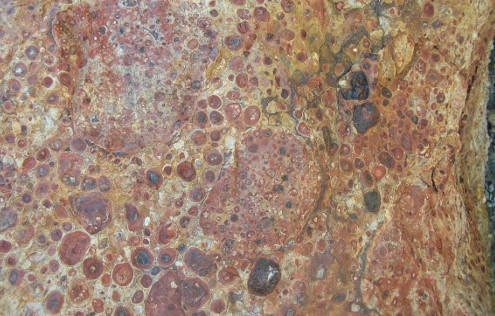
.jpg)
The principal ore of aluminum is bauxite, a complex mixture of a number of aluminum hydroxides and hydrous aluminum oxides. The most common aluminum-bearing minerals in bauxite are gibbsite (AlOH3), boehmite (AlO(OH)), and diaspore (AlO(OH)). In the Arkansas deposits, diaspore has not been reported. Free quartz along with iron and titanium oxides are common components. Bauxite ranges in color from off-white to deep reddish brown, and structurally from a soft earthy material to a well-cemented rock. An easily recognizable oolitic (BB-sized concretions) to pisolitic (pea-sized concretions) grain texture characterizes many bauxite deposits, including those in Arkansas. Commercial bauxite usually has a minimum content of 50 to 55 percent alumina (Al2O3).
In the bauxite refining process, the aluminum-bearing minerals in bauxite are converted in a multiple-step process to alumina (Al2O3). Alumina can be smelted to form metallic aluminum or it can be used as the source of many other products, including refractory materials used to line high-temperature rotary kilns and metallurgical furnaces. In addition, alumina is a source of many chemicals used in the paper and ceramic industries, in petroleum refining, and in some water-purification processes. Other uses are for the production of synthetic corundum for the manufacture of abrasive stones and grinding wheels, as propants in the petroleum-production industry, and as an ingredient in deodorants, antacids, and some medicines.
Mining and Geology of Arkansas Bauxite
Many of the early-mined Arkansas bauxite deposits were exposed on the surface as outcrops or were beneath only a thin layer of sediments. Consequently, surface-mining methods were initially the most practical and economical. Before and during World War II, significant tonnages were mined underground. Some years after the war, surface operations resumed. Open-pit panel mining has been the normal surface method since the early 1960’s. A strip or block of bauxite is exposed, mined, and then another panel is exposed. The first panel is normally refilled with waste rock. Several panels may be open at the same time to supply the proper blend of ores to meet the mill specifications. In recent years, major reclamation programs have begun to restore not only the recently mined land, but much of the land that was disturbed before reclamation laws went into effect.
The Arkansas bauxite region covers about 275 square miles in the northern part of the West Gulf Coastal Plain and is divided into two mining districts. One area is in Pulaski County south and east of Little Rock and the other is in nearby Saline County, northeast and east of Benton. The bauxite is present mostly as sheet or blanket deposits in very close proximity to outcrops of the intrusive igneous rock, nepheline syenite. The deposits formed in early Tertiary time, developing as soils along the western edge of a shallow marine basin that occupied the Mississippi River Embayment. During that time, hills and knobs of syenite as islands were exposed to intense chemical weathering in a tropical or near-tropical environment (lateritic weathering). In the weathering process, leaching by rain, ground water, and perhaps by salt spray, decomposed the original igneous rock minerals (feldspar and nepheline), removed much of the silica, and concentrated the newly formed oxides and hydroxides of aluminum as the rock we term bauxite. These are residual deposits because they formed essentially in place (in situ paleo-soils). Many other deposits, generally smaller, consist of bauxite removed by erosion from its site of origin and redeposited nearby (transported deposits).
History of Discovery and Production
The aluminum industry has contributed significantly to the state's economy for many years. Bauxite was first mined in Arkansas as an ore of metallic aluminum in 1898, only 11 years after John C. Branner, State Geologist, first identified it in a sample from Pulaski County. Over the years, Arkansas industry has remained the major producer in the United States, providing about 90 percent of all domestic tonnage mined. As aluminum became more widely available, many new uses of the metal (and of the by-products of the aluminum industry) were discovered, and consumption increased rapidly. Tonnages of bauxite mined in Arkansas increased much more slowly than national consumption because larger deposits supplying higher grade bauxite were readily available in the Caribbean region. In the early stages of World War II, merchant freighters carrying bauxite to the United States suffered high losses to enemy submarines. It was imperative that foreign supplies be supplemented by increased domestic production. The tonnage of bauxite mined in Arkansas quickly increased many fold to meet wartime demands for aluminum, which was especially critical to the military aircraft industry. In 1943, more than 6 million long tons of bauxite were mined. Because of changing domestic and world economic market conditions, 1982 was the last year in which bauxite was mined in Arkansas for aluminum metal. Small tonnages continue to be mined and used in the production of a variety of alumina-based materials, including various chemicals, abrasives, and propants.
References
Bramlette, M. N., 1936, Geology of the Arkansas bauxite region: Arkansas Geological Survey Information Circular 8, 68 p.
Canby, T. Y., 1978, Aluminum, the magic metal: National Geographic, v. 154, no. 2, p. 186-211.
Gordon, MacKenzie, Jr., Tracey, J. I., Jr., and Ellis, M. W., 1958, Geology of the Arkansas bauxite region: U. S. Geological Survey Professional Paper 299, 268 p.