
Water is a compound composed of the two elements hydrogen and oxygen. This amazing substance can exist near the earth’s surface in all three physical forms: as a liquid, vapor or gas and as a solid. The humidity in the air is a measure of the amount of water vapor suspended within it. When water vapor condenses it forms droplets (up to 20,000 in a cubic inch) that in large amounts form clouds and fog. Further condensation causes the droplets to grow so large that they then fall to earth as rain. If the air temperatures fall below 32 degrees F or 0 degrees C then the droplets solidifies (freezes) and forms snow, sleet or hail. Because of this ability the earth’s water passes through what is called the hydrologic cycle this model demonstrates the movement of water from three worldwide reservoirs- the atmosphere, the surface, and the earth’s crust. Water has very important chemical and physical properties that make it so very special for human beings, all known living things and even to shaping the surface of the earth.
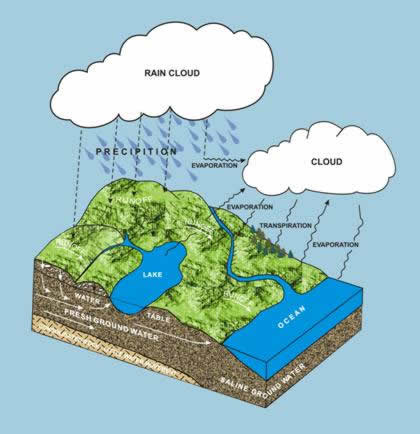
The water cycle. A schematic portrayal of the continual movement of water in the atmosphere, on the earth’s surface, and beneath its surface.
Water's Chemical Properties
Water’s chemical formula is H2O. The water molecule odd shape with both hydrogen atoms occurring on the same side of the oxygen atom gives water its ability to “stick” to itself and to other surfaces. The hydrogen atoms create a positive electrical charge while the oxygen atom creates a negative charge. The attraction to one another is what causes water to form droplets. The chemical properties make water essential to the functioning of living things including human beings. We must ingest or drink water in order to maintain good health
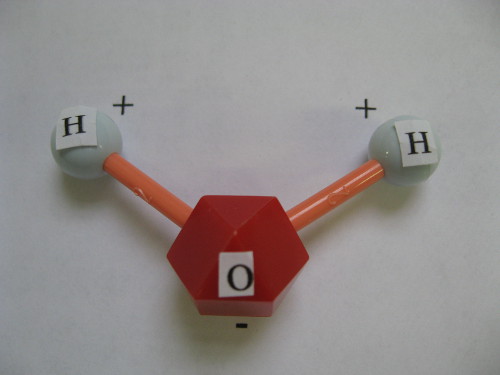
Water molecule
This model of a water molecule shows the arrangement of one oxygen atom bound to two atoms of hydrogen and their positive and negative charges.
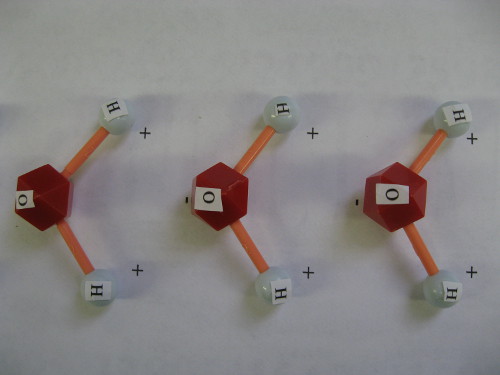
Arranged water molecules
Arranged water molecules positive to negative charges make water “sticky” and from drops or beads on a smooth surface.

Water beads
Water beads on hood of a car because of the arrangement of water molecules.
Water's Physical Properties
Being able to exist in all three physical states on the earth surface makes water a one of a kind substance that is liquid, solid (ice), at 32 F and gas (steam) at 212 F. Water is unique in that it is one of the very few substances that when it becomes a solid it becomes less dense and will float on its liquid form. Water can store a lot of heat energy because it has a high specific heat index. This allows large bodies of water to help cool or heat the earth’s atmosphere therefore keeping our climate’s temperature more constant. Because water has mass or weight (8.33 pounds per gallon), that when it is in motion it can move with great force as in the current of a river or as waves in the ocean. Such forces shape the land on the earth’s surface.
The most uncommon form of water found in Arkansas is as snow or ice. Photo of a light covering of snow on Carrion Crow Mountain at the town of Atkins, Pope County, Arkansas.

References
Howard, J.M., Colton, G.W., and Prior, W.L., eds., 1997, Mineral, fossil-fuel, and water resources of Arkansas: Arkansas Geological Commission Bulletin 24
Arkansas has abundant water resources due to its geographic location. Arkansas receives an average of 49 inches of rainfall a year or approximately 124 billion gallons per day (bgd). Streams and rivers that flow into the state add an additional 37 bgd as surface water. However at least half of this total always returns to the atmosphere as vapor and some 48 percent becomes surface runoff, with most of the this going into the Mississippi River (See Surface Water). Only about 4 percent or 2 inches out of the 49 inches of rain that falls on Arkansas finds its way into the ground to become what is referred to as ground water. The average resident of Arkansas uses about 160 gallons of water per day with the state as a whole is using close to 11.5 billion gallons of water per day.
Water, one of our most important resources, is taken for granted unless we have too much or too little of it.

References
Holland, T.W., 2007, Water use in Arkansas, 2005: U.S. Geological Survey Scientific InvestigationsReport 2007-5241
Howard, J.M., Colton, G.W., and Prior, W.L., eds., 1997, Mineral, fossil-fuel, and water resources of Arkansas: Arkansas Geological Commission Bulletin 24